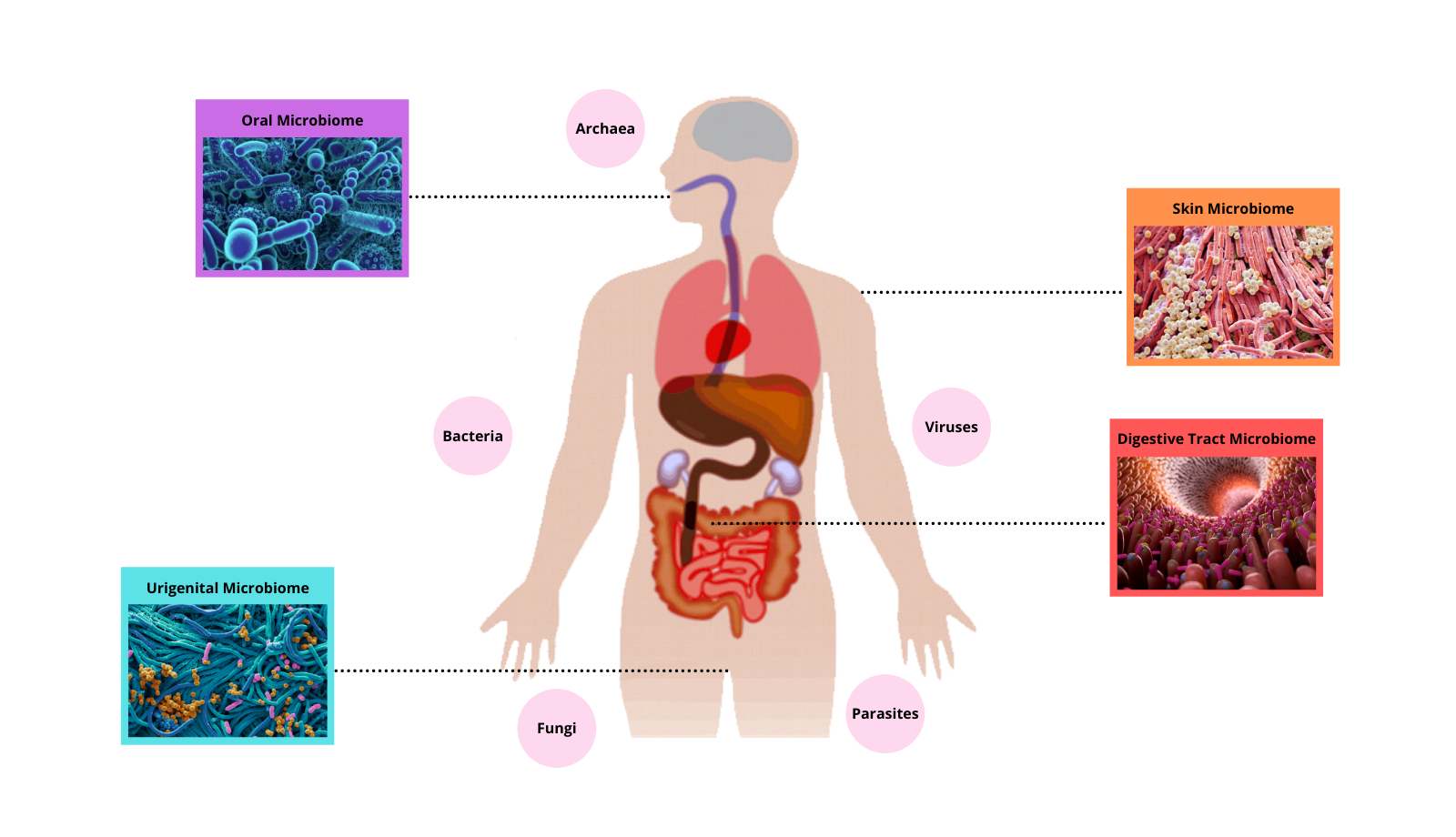
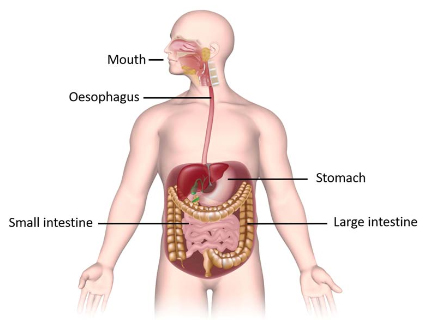
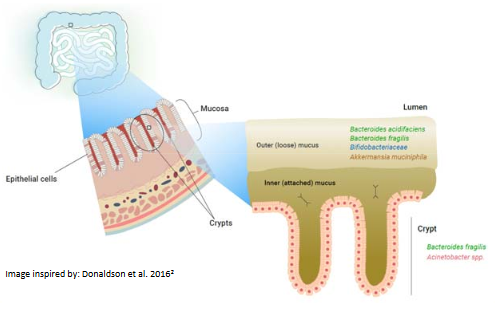
Microorganisms are everywhere – in, on and around us! Amazingly, the number of microbes that live in the human body are more plentiful than the number of our own body cells! Different regions of our body are colonised by different communities of microbes, most of which are harmless or beneficial to us – as long as we provide them with a healthy environment to live in. The community of microbes that live in our lower gastrointestinal tract, collectively termed the ‘gut microbiome’, play a vital role in maintaining our overall health. It is comprised of bacteria, fungi, protists and viruses. Food that is not absorbed in the small intestine passes through to the colon (large intestine) where the gut microbes feast on it, producing metabolites that are then absorbed into our bodies.
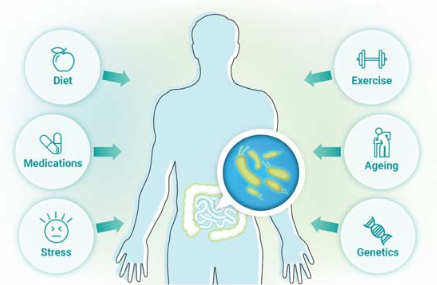
Our gut microbiome, while predominantly similar to others around us, is in fact unique to our own body and is ever changing depending on what it is exposed to. Diet, genetics, medications, exercise, stress and aging all influence the make up of our gut – for better or for worse! A healthy microbiome plays a vital role in nutrient and mineral absorption, provides us with essential enzymes, vitamins and amino acids, helps regulate mood and shapes our immune system. An unhealthy microbiome – termed ‘dysbiosis’ – has been demonstrated to drive inflammatory disease, autoimmune disease, weight issues, mental health issues, cancers, metabolic disease and neurodegenetive disease. So, it’s important we consider the health of our gut as a major part of our wellness journey.
How can we maintain a healthy microbiome? We aim to support beneficial microbes and their metabolites, while reducing those that are detrimental – begin with feeding ourselves well! The foods that we eat drive changes in the microbial species that live in our gut, for example – a high sugar diet leads to overgrowth of sugar-loving microbes that can produce metabolites that are harmful to our body, while those that live on fibre are more beneficial. A wide and varied diet that is full of goodness results in a rich and diverse microbiome in the gut, ultimately leading to better health for our whole body! Supplementation with good quality pre- and pro-biotics can boost beneficial bacterial species, and aiming for an active lifestyle where stress is minimized also has significant impact on our gut health.
Our in-depth module ‘Gut Health and the Microbiome’ provides a wealth of information on how you can shape your diet and lifestyle to promote a healthy gut, including specifics on which foods significantly impact health or disease, and the latest science on lifestyle and environmental factors that influence our gut microbiome. Combined with the analytic report of your microbiome testing, these valuable insights can be pivotal to help you better understand what your body needs to move forward in your health journey.